RCH Achieves AWS Life Sciences Competency
Leading Cloud solutions exclusively for Life Sciences teams that accelerate discovery, optimize costs and ensure scalability and compliance in AWS.
In the rapidly evolving world of Biotechnology and Life Sciences, the ability to decode the intricate sequences of DNA has transformed how we understand genetics, disease, and even evolution. Gene sequencing, the process of determining the precise order of nucleotides in a DNA molecule, is at the heart of many breakthroughs in medicine and diagnostics. At RCH Solutions, we are proud to support these innovations with our cutting-edge Bio-IT and scientific computing services, enabling the full potential of gene sequencing for our partners in the Life Sciences industry.
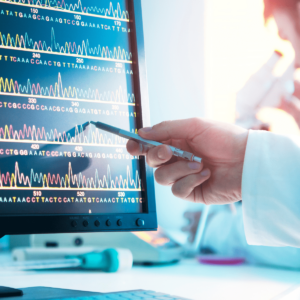
Gene sequencing is no longer a distant research tool; it has become a fundamental technology used across a wide range of applications, from diagnosing rare genetic disorders to understanding complex diseases like cancer. The data derived from sequencing a genome offers critical insights into the genetic makeup of organisms, providing a roadmap for understanding biological functions and disease mechanisms.
Gene sequencing has opened the door to personalized medicine in Healthcare, where treatments are tailored to a patient’s unique genetic profile. This approach transforms how we treat diseases, making therapies more effective and reducing the risks of adverse reactions. Furthermore, gene sequencing allows for early detection of diseases, enabling proactive management and even prevention in some cases.
Given its wide-reaching applications, gene sequencing has become an indispensable tool in Life Sciences. Yet, it brings forth significant challenges—particularly in the immense volume of data generated and the computational resources required to process, analyze, and interpret the data.
At RCH Solutions, our advanced and scientific computing services support the end-to-end gene sequencing process, helping our customers maximize the value of their genetic data. Our team understands the unique demands of gene sequencing workflows and provides tailored solutions to meet the challenges of data management, storage, and analysis.
 Gene sequencing generates massive amounts of data, especially with modern sequencing techniques like Next-Generation Sequencing (NGS), which can sequence entire genomes in hours. This data must be processed and analyzed efficiently, requiring specialized high-performance computing (HPC) environments. At RCH Solutions, we leverage the latest HPC technologies to provide scalable computing resources that handle these data-intensive workflows.
Gene sequencing generates massive amounts of data, especially with modern sequencing techniques like Next-Generation Sequencing (NGS), which can sequence entire genomes in hours. This data must be processed and analyzed efficiently, requiring specialized high-performance computing (HPC) environments. At RCH Solutions, we leverage the latest HPC technologies to provide scalable computing resources that handle these data-intensive workflows.
Our HPC services are designed to accelerate data analysis, helping scientists quickly identify genetic variations, mutations, and other critical insights that inform research and clinical decision-making. Optimized algorithms and software frameworks help our team ensure that large sequencing datasets are processed swiftly and accurately. This reduces the time it takes to move from raw data to actionable insights.
Gene sequencing’s storage and processing needs are often unpredictable, especially in research environments where data is continuously generated. RCH Solutions offers Cloud-based computing solutions that provide the scalability and flexibility required to handle these demands. Whether customers need temporary processing power for large sequencing projects or long-term storage for genomic data, we ensure they have the right resources when needed.
With Cloud-based platforms, Life Sciences organizations can access the necessary storage capacity, ensuring that sequencing data is safely stored and readily accessible for analysis. When properly architected and governed, this capability supports compliance with data security standards and regulatory requirements, a crucial factor in the Life Sciences sector.
Gene sequencing often involves sensitive information, particularly in clinical applications. At RCH, we prioritize the security of this data. Our solutions are built with industry-leading encryption protocols and adhere to the highest compliance standards, including GxP, ensuring that genomic data is securely stored and transmitted.
Additionally, we help customers implement data governance strategies, ensuring all genomic data is properly indexed, traceable, and organized according to best practices. This enables Life Sciences organizations to maintain the integrity of their data while supporting downstream analyses.

One of the critical challenges of gene sequencing is the ability to make sense of vast amounts of genetic data. At RCH Solutions, we integrate advanced analytics tools that allow researchers to analyze and interpret sequencing results more effectively. Our team combines computational biology expertise and cutting-edge data analytics tools to help scientists identify patterns, correlations, and anomalies within complex genomic datasets.
We also integrate data from diverse sources, such as clinical trials, patient records, and research datasets. This enables more comprehensive analyses that drive better insights into genetic diseases, personalized treatments, and evolutionary processes.
Collaboration is key in the fast-paced world of gene sequencing and genomics. Our team at RCH Solutions works closely with our customers to understand their specific challenges and goals, offering personalized support and strategic guidance throughout the sequencing process. From the initial stages of data acquisition and storage to the final analysis and interpretation, we ensure that our customers have the proper infrastructure, resources, and expertise to achieve their objectives.
As the Life Sciences industry evolves, gene sequencing remains a cornerstone technology that powers personalized medicine and disease research breakthroughs. At RCH Solutions, we are proud to provide the Bio-IT infrastructure and scientific computing services that support this critical work. Whether it’s through high-performance computing, Cloud-based solutions, data security, or advanced analytics, our team is committed to helping Life Sciences organizations harness the full potential of gene sequencing.
By partnering with RCH Solutions, you gain access to the tools, technologies, and expertise needed to drive innovation and ensure that your genomic research and applications reach their fullest potential. Let us help you decode the future of Life Sciences with advanced computing solutions tailored to the needs of your unique gene sequencing landscape.
In the Life Sciences, analyzing vast datasets like genomic sequences or clinical trial results is routine. Ensuring the security and integrity of this data is crucial, especially under tight regulatory oversight.
Amazon Web Services (AWS) provides a platform with certified experts that cater to these needs. AWS offers tools that help streamline data processes, meet compliance standards, and safeguard intellectual property. The key is to use these tools efficiently to maintain data integrity and confidentiality.
AWS solutions are designed to meet the specific demands of Life Sciences, such as upholding GxP compliance and guarding sensitive patient data. By aligning with these requirements, organizations can adhere to regulatory standards while tapping into the benefits of Cloud technology. Moreover, AWS’s voluntary participation in the CSA Security, Trust & Assurance Registry (STAR) Self-Assessment showcases its transparency in compliance with best practices, establishing even more trust for users.
AWS’s commitment to integrating compliance controls means it’s woven through the entire system, from data centers to intricate IT processes. For example, when handling genomic data, AWS ensures encrypted storage for raw sequences, controlled access for processing, and traceable logs for any data transformations, all while adhering to regulatory standards.
This tailored AWS infrastructure offers Life Sciences organizations unparalleled control. Imagine researchers working on a groundbreaking vaccine. As they collect vast amounts of patient data, they need a system that can not only securely store this information but also track every modification or access to it.
With AWS, an automatic log is generated each time a researcher accesses or modifies a patient’s record. This means that six months later, if there’s a question about who made a specific change to a patient’s data, the researchers can quickly pull up this log, verifying the exact date, time, and individual responsible. Data management on AWS is about ensuring data is traceable, consistent, and always under the organization’s purview.
Life Sciences data, whether it’s genomic sequences or computational studies, needs robust security. This data’s sensitivity and proprietary nature mean any breach could harm research outcomes, patient confidentiality, and intellectual property rights.
To tackle these concerns, AWS provides:
Navigating the maze of regulatory requirements in the Life Sciences sector can be daunting. AWS offers tools designed specifically to ease this journey.
AWS Artifact stands out as it provides on-demand access to AWS’s compliance reports. With information at their fingertips, companies can confidently maintain regulatory compliance without the traditional runaround of audit requests.
Further strengthening the compliance arsenal, AWS Config offers a dynamic solution. Rather than periodic checks, AWS Config continuously monitors and records the configurations of AWS resources. For instance, if a Life Sciences firm were to deploy a genomic database, AWS Config would ensure its settings align with internal policies and external regulatory standards. Utilizing machine learning, AWS Config can also predict and alert potential non-compliance issues before they become critical.
This continuous oversight eliminates gaps that might arise between audits, allowing for consistent adherence to the best practices and regulatory norms.
AWS provides a solid foundation for data management and compliance, but sometimes, specific industries demand specialized solutions. This is where third-party tools like Turbot come into play. It’s tailored for sectors such as Life Sciences thanks to its real-time automation, governance, and compliance features.
Consider a pharmaceutical company conducting clinical trials across multiple countries, each with unique compliance criteria. Turbot ensures that every AWS resource aligns with these diverse regulations, minimizing non-compliance risks.
Beyond mere monitoring, if Turbot detects any discrepancies or non-compliant resources, it immediately rectifies the situation without waiting for human intervention. This proactive approach ensures robust security measures are consistently in place.
Life Science industries operate within complex regulatory landscapes, and handling vast datasets requires meticulous attention to security, traceability, and compliance. AWS’s platform is designed to cater to these needs with sophisticated tools for data governance, security, and audit preparedness, however, without a specialized Life Sciences scientific computing provider with deep AWS expertise, like RCH, you may be leaving AWS opportunities and capabilities in the balance. To maximize the potential of these tools and navigate the intricate junction where science and IT overlap in the AWS Cloud, and beyond, a subject matter expert is crucial.
Contact our certified AWS experts to empower your research with specialized Bio-IT expertise and help streamline your journey to groundbreaking discoveries.
Sources:
https://aws.amazon.com/solutions/health/data-security-and-compliance/
https://aws.amazon.com/health/solutions/gxp/
http://www.rchsolutions.com/cloud-computing/
https://aws.amazon.com/health/genomics/
https://aws.amazon.com/solutions/case-studies/innovators/moderna/
https://aws.amazon.com/compliance/data-center/controls/
https://aws.amazon.com/blogs/aws/new-aws-config-rules-now-support-proactive-compliance/
https://turbot.com/guardrails/blog/2018/11/healthcare-and-life-sciences
https://turbot.com/guardrails/blog/2018/04/gartner-cspm
http://www.rchsolutions.com/resource/elevated-perspectives-security-in-the-cloud/